Drug screens
Beyond enabling progress in fundamental biology, WBI enables drug screens in disease models that allow for tracking the effects at multiple spatial scales from cells to the whole body and at temporal scales from seconds to days. The majority of drug-screening workflows depend on observing a subset of drug-induced phenomenology such as the molecular binding properties1, the effects on individual cells or subset of tissues2 or influence on behavior3,4. WBI offers a complementary lens through which to observe the impact of drugs and genetic interventions on the entire body, enabling the identification of unanticipated off-target consequences in organ systems not under direct study, as well as the discovery of unexpected benefits in tissues not previously considered. It would also reveal outcomes arising from system-wide interactions that could be missed when studying cells, tissues, or organs individually, rather than collectively within their native whole-body environment. Ultimately, combining WBI with fictive-swimming virtual-reality systems5 will enable research at the intersection of whole-body cellular physiology, systems neuroscience, and behavior, shedding light on the interactions between interoception and exteroception, as well as the interplay and feedback between cognition and visceral state in driving behavior and physiology.
Example: WBI identifies new cellular response properties to Ketamine
As a demonstration of using WBI for drug screening, we performed a WBI screen for cells that respond to ketamine, a dissociative anesthetic. Quantifying the strongest responding cells by ranking Fnrom increases, we identified a functional compartment that aligns with the outer perimeter of the brain, likely corresponding to the brain meninges, which is responsive to ketamine. While the effects of ketamine on neurons and glia are documented6, the role of the meninges is less studied; meninges have not yet been functionally imaged in any species in vivo7 and remain largely uncharacterized in zebrafish. With no existing selective driver line8, they are difficult to segment based on anatomy due to the thinness of the tissue. This highlights how WBI activity analysis is able to delineate functional compartments that are otherwise challenging to label – i.e., identify, within the many cells in the body, small groups of cells with specific dynamics that would otherwise remain undetected.
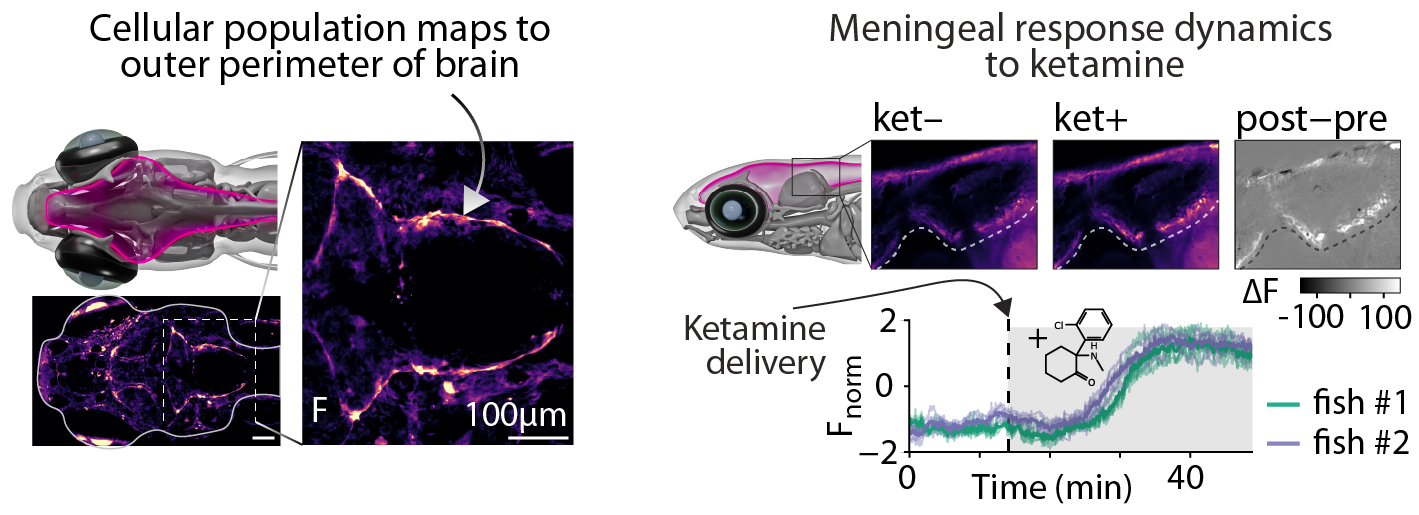
Meningeal response to ketamine exposure. Animals were exposed to ketamine following a 25 min period of baseline imaging. Top) Sagittal section showing pre- and post-exposure to ketamine; Bottom) activity traces from meningeal regions.